Functional and Disease Assessment
Last updated: November 9, 2014
Synonyms: American College of Rheumatology functional classification, Arthritis Impact Measurement Scale (AIMS), Health Assessment Questionnaire (HAQ), Modified Health Assessment Questionnaire (mHAQ), multidimentional HAQ (MD-HAQ), Bath Ankylosing Spondylitis Activity Index (BASDAI), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC); Systemic Lupus Erythematosus Disease Activity Index (SLEDAI).
CPT Code: RA functional status assessed 1170F; administration and interpretation of health risk assessment instrument 99420
Description: A variety of validated scales have been developed to assess the functional status of patients with arthritis. There are three main types: (a) assessment of functional capacity by either patient self-report or physician; (b) quantitative joint examinations; (c) measurement of performance. Although these assessment tools have been applied primarily in clinical trials of new drugs and evaluation of disability status, they are now being used in clinical practice where functional and clinical outcomes may be easily measured, recorded, and studied longitudinally.
Assessment of Capacity: The American College of Rheumatology has devised a four-class scale to determine the global functional status, primarily in patients with RA. This physician-determined scale is based on the patient’s ability to perform activities of daily living including self-care and vocational and avocational activities (Table A).
Global scales measuring pain or overall functional status may be determined using a 10-cm visual analog scale ranging from no problems (score = 0 cm) to very severe limitations (score = 10 cm). These are often used in clinical trials but can be applied to numerous diseases and situations and can be independently completed by the patient or physician or both.
The most established self-report scale is the Health Assessment Questionnaire (HAQ). A total of 20 items regarding activities of daily living are scored by the patient on a scale of 0 (no problem), 1 (some difficulty), 2 (much difficulty), or 3 (extreme difficulty or unable to do). A shorter modified version of the HAQ, the Modified Health Assessment Questionnaire (mHAQ) (Table B), with eight questions also scored from 0 through 3, has been advocated as easier to perform in usual clinical situations. The mHAQ score is the mean value of these eight questions (range: 0-3). This scale has great utility when applied to RA patients in both clinical trials and practice settings.
The Arthritis Impact Measurement Scales (AIMS) involves a longer questionnaire and has been validated in both RA and psoriatic arthritis. Patients with relatively low educational levels may have difficulty completing this scale without assistance.
A more generalized assessment of overall health is the Short-Form 36 Health Status Questionnaire (or SF-36), which can be administered in person orby telephone and covers general aspects of function in life activities, including social interactions. It has been used in large studies assessing health care delivery as well as in evaluating responses to new treatments in clinical trials.
Various disease-specific questionnaires have been developed. For instance in lupus, the SLE Disease Activity Index (SLEDAI) and Systemic Lupus Activity Measure (SLAM) are two indices of activity and severity that have also been used in clinical trials. A questionnaire for patients with fibromyalgia, the Fibromyalgia Impact Questionnaire (FIQ), has been validated and tested in small studies. In those with osteoarthritis of the knee, the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) is a commonly used, validated functional assessment tool. For ankylosing spondylitis trials, the Bath Ankylosing Spondylitis Activity Index (BASDAI) also uses patient-derived questionnaires to assess activity.
| Table A: 1991 American College of Rheumatology Functional Classification in Rheumatoid Arthritis | ||||
| Class | Description | |||
|---|---|---|---|---|
| I | Able to perform all activities of daily living | |||
| II | Able to perform activities of daily living and vocational activities; limited in avocational activities | |||
| III | Able to perform activities of daily living but not vocational or avocational activities | |||
| IV | Limited in all activities, including self-care | |||
| Table B: Modified Health Assessment Questionnaire (mHAQ) | ||||
| Please check the column that is the best answer for your abilities at this time: | ||||
| At this moment, are you able to : | Without any difficulty (0) | With some difficulty (1) | With much difficulty (2) | Unable to do (3) |
| Dress yourself, including tying shoelaces and doing buttons? | ||||
| Get in and out of bed? | ||||
| Lift a full cup or glass to your mouth? | ||||
| Walk outdoors on flat ground? | ||||
| Wash and dry your entire body? | ||||
| Bend down to pick up clothing from the floor? | ||||
| Get in and out of a car? | ||||
| Turn regular faucets on and off? | ||||
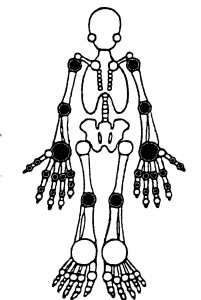
Quantitative Joint Examination: Counts of tender and swollen joints are a standard means for measuring disease activity and response to therapy in patients with RA. These indices can also be applied to other forms of arthritis, including psoriatic arthritis, osteoarthritis and lupus arthritis. A comprehensive joint examination would include 66 and 68 joints. scored for swelling and tenderness, respectively. A more common practice in both clinical trials and rountine care is to quantitatively assess 28 joints – the PIPs, MCPs, Wrists, Elbows, Shoulders and Knees (see figure). Studies suggest that evaluation of fewer accessible joints (e.g., the 28 joint exam) provides as much information as a full 66/68 joint exam. A joint is assigned a value of 1 if abnormal (tender or swollen) and 0 if normal. The sum of these is called the tender joint count (TJC) and swollen joint count (SJC). Although seldom used in clinical trials the “joint score” can be used to reflect the severity and extent of joint involvement and is the sum of the individual joints scored on a scale of 1 to 3. Joint pain is scored as 0 (no pain), 1 (tenderness), 2 (tenderness with wincing), or 3 (wince and withdrawal). Joint swelling is scored as 0 (none), 1 (swelling, just appreciable), 2 (swelling within normal joint contours), or 3 (swelling outside of normal joint contours).
Rheumatoid Arthritis: There are numerous tools that can be employed both in outpatient daily care and clinical trials (Table C). These are either A) patient derived functional (or quality of life) surveys; B) Patient or physician derived assessment of disease activity or C) Drug or disease responses.
| Table C. Functional* and Disease Activity+ Measures in Rheumatoid Arthritis | |
| HAQ* | 20 questions of daily activities; scored 0-3 |
| mHAQ or HAQ-DI* | 8 or 10 questions of daily activities; scored 0-3; MD-HAQ adds 2 more questions on “getting in/out of a car” and “can you participate in recreational activity/sport” |
| SF-36* | Measures physical function, activity limits due to physical problems, bodily pains general health perceptions, vitality, social functioning, mental health or emotional difficulties (score range 0-100; lower = poorest) |
| VAS (0-10cm scale)+ | Scored on a 10cm or 100 mm scale; used to assess patient pain (in the last week), patient or physician global assessment (MD Global) |
| ACR Response+ | ACR20 = >20% improvement in TJC AND SJC; with >20% improvement in 3 of these 5 measures: patient pain, patient global, MD global, HAQ (functional) or an acute phase reactant (ESR or CRP). ACR50 and ACR70 utilize the same formula but require >50% or >70% respectively |
| ACR Remission+ |
In clinical trials: TJC, SJC, patient global assessment AND CRP < 1 (all on 0-10 scale; CRP as mg/dl) OR SDAI < 3.3 In practice: TJC, SJC, AND patient global assessment < 1; or CDAI < 2.8 |
| DAS28 (ESR)+ | 0.56 x √(TJC) + 0.28 x √(SJC) + 0.70 log(ESR) + 0.14 x Global Health (patient) |
| SDAI+ | (using the 28 joint exam) TJC + SJC + patient global assessment (cm) + MD Global (cm) + CRP (mg/dl) (range 0-100~) |
| CDAI+ | (using the 28 joint exam) TJC + SJC + patient global assessment (cm) + MD Global (cm) (range (0-76) |
| RAPID3+ | MD-HAQ (0-3) x 3.33 + patient global assessment (cm) + patient pain (cm) (range 0-30) |
| GAS+ | Patient pain (cm) + raw mHAQ (0-24) + TJC (range 0-62) |
Assessment of Performance: In RA, the walking time (for 25 or 50 ft) and grip strength (measured using a modified sphygmomanometer) are uncommonly used functional measures that show correlations with other measures of disease status. These are less reliable and reproducible compared to functional assessments like the HAQ.
Confounding Factors: Most validation studies of questionnaires have been done in white, English-speaking populations. Their applicability to those who are non-English speaking or of low educational levels cannot be assumed. However, some forms have been validated in Spanish and French.
Indications: These assessments have been used in trials of new therapeutic agents, especially in patients with RA. Applications in other diseases such as SLE or fibromyalgia are less well established. Insurance companies, other third-party payers, and disability assessment organizations have begun to use these outcome and disease measures as a means of ascertaining the appropriateness of using or continuing certain, novel and expensive therapies . The routine use of these outcome, activity and functional measures in clinical practice has grown steadily; prompted by treat-to-target studies and the desire to objectify treatment decisions.
Cost: Time is the major cost involved to be incurred by the patient and practitioner. A 28 joint exam can be done in <3 minutes and the 8-10 question versions of the HAQ can be done in minutes by most patients. Some practioners have these questionnaires completed either online or in advance of the medical visit. A computerized data entry system may facilitate longitudinal and comparative analyses.
BIBLIOGRAPHY
Hochberg MC, Chane RW, Dwosh I, et al. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum 1992;25:498–502. PMID: 1575785
Pincus T, Brooks RH, Callahan LF. Prediction of long-term mortality in patients with rheumatoid arthritis according to simple questionnaire and joint count measures. Ann Intern Med 1994;120:26–34. PMID:8250453
Smolen JS, Aletaha D. The assessment of disease activity in rheumatoid arthritis. Clin Exp Rheumatol. 2010;28:S18-27. PMID: 20576221